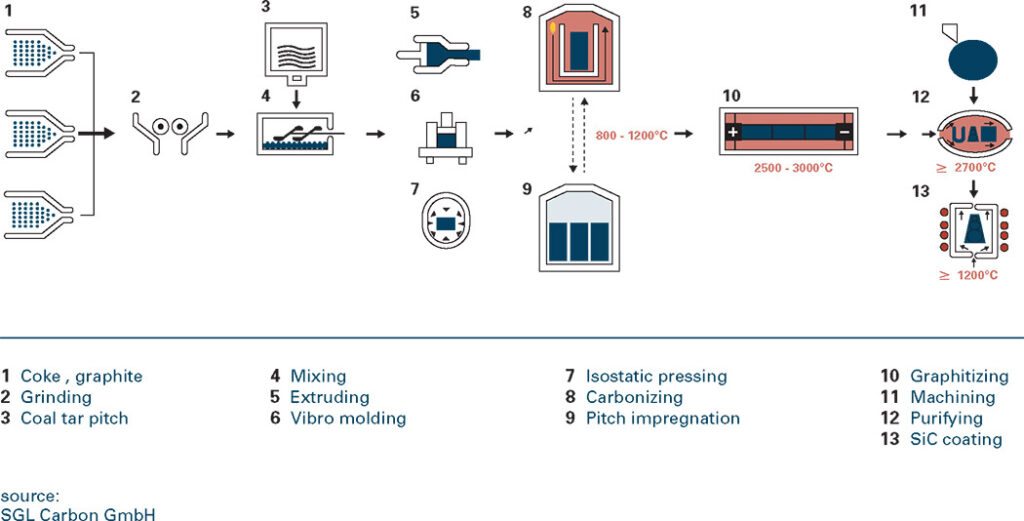
What is graphite?
History
CHEMISTRY
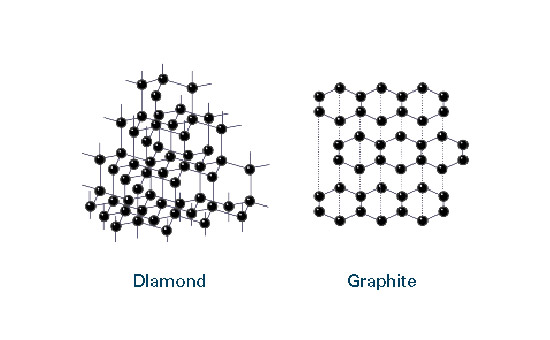

Graphite Properties
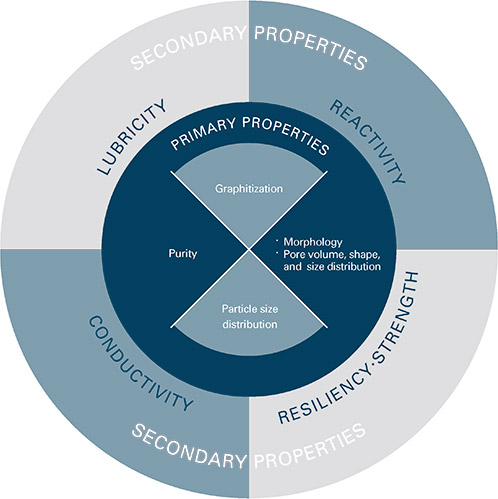
Types of graphite
Graphite is formed naturally through the metamorphism of carbon rich materials in rock which leads to the formation of either crystalline flake graphite, fine grained amorphous graphite, or crystalline vein or lump graphite.
Amorphous Graphite
Amorphous graphite is the most abundant form of graphite. It is generally a soft, darker black colored graphite, less reflective than other varieties of natural crystalline graphite. Commonly found as “microcrystalline” particles, this variety of graphite has a lower graphitic carbon level when compared to other naturally occurring crystalline graphite varieties. Amorphous graphite is extracted using conventional coal-type mining techniques. Due to its softness, including as an additive to metallic alloys, it can be used as a lubricant or grease in many applications.
Amorphous Graphite

Amorphous Graphite
Flake graphite is the most recognizable natural graphite material. It is commonly used in lead pencils and is desirable for emerging technologies such as lithium-ion batteries. Flake graphite is found in metamorphic rock and can have a carbon range of 85-98%. Depending on the degree of weathering of the ore rock, flake graphite is mined using standard hard or soft rock mining techniques. Unlike other varieties of natural crystalline graphite, flake needs to be mechanically/chemically processed or “upgraded” to reach the necessary carbon levels for industrial applications.
Crystalline Flake Graphite

Crystalline Vein Graphite
Typical veins measure from centimeters to nearly two meters in thickness with the highest purity material being located toward the center of the vein away from contact with the wall rock. Vein graphite is mined using conventional shaft or surface methods typically used to mine vein-type deposits.
In many applications vein graphite may offer superior performance since it has slightly higher thermal and electrical conductivity, which result from its high degree of crystalline perfection. Vein graphite also has the highest degree of cohesive integrity of all natural graphite materials. High cohesive “energy” means that vein graphite is easy to mold and can be formed into solid shapes without the aid of a binder addition.
Crystalline Vein Graphite

Synthetic Graphite
Synthetic graphite, sometimes referred to as artificial graphite, is found in two forms: Primary Synthetic, and Secondary Synthetic. Primary Synthetic is produced through the high temperature treatment of specific coke precursors. Secondary Synthetic is the by-product of graphite electrode and part manufacturing. They have a dark gray to black, flat appearance. Manufactured from calcined cokes, and graphitized at temperatures approaching 3000°C, these materials offer high purity, excellent lubrication, and electrical conductivity. Synthetic graphite is used in many applications including but not limited to friction, foundry, electrical carbons, fuel cell bi-polar plates, coatings, electrolytic processes, corrosion products, conductive fillers, rubber and plastic compounds, and drilling applications.Synthetic Graphite
Occurrence & production of graphite
Graphite is a non-metal but has many properties of metals. It is an excellent conductor of heat and electricity and has the highest natural strength and stiffness of any material. It maintains its
strength and stability to temperatures in excess of 3,600°C and is very resistant to chemical attack. At the same time it is one of the highest of all reinforcing agents and has high natural lubricity.